Developing New Technology for the Encapsulation of mRNA-Based Vaccines in Lipid Nanoparticles (LNPs)

The COVID-19 pandemic has necessitated the fast enhancement of new systems for vaccine enhancement and delivery. A person vital progress has stemmed from the require for largescale production of mRNA-based mostly vaccines encapsulated in lipid nanoparticles (LNPs), enabling fragile mRNA-dependent vaccines to much better integrate into patients’ cells.
KNAUER has been at the forefront of this crucial growth in the struggle in opposition to COVID-19, with the company’s Impingement Jets Mixing engineering supplying a system for the higher stream production of lipid nanoparticles.
We not long ago spoke to Dr. Paul Pietsch from KNAUER about the company’s track record, its selection of specialisms and its pioneering function to facilitate the largescale production of LNPs though meeting the stringent restrictions and exacting demands of the biopharmaceutical industry.
You should could you demonstrate what KNAUER can provide to engineers and marketplace specialists?
KNAUER has been manufacturing large-top quality scientific devices considering that 1962. We are an knowledgeable partner in the supply of products for use in the pharmaceutical marketplace, this kind of as liquid chromatography techniques and technology for lipid nanoparticle creation and encapsulation.
We are happy to have many specialists in our team, enabling us to create progressive and particular person options built all over every of our customers’ distinctive worries. In addition to this, we present engineering solutions to build devices that meet the exact requirements and specifications of our close end users.
What can KNAUER provide the pharmaceutical market that its competition, most likely, cannot?
There are numerous companies out there giving gear and units to the pharmaceutical industry, and they appear in several shapes and dimensions on the other hand, KNAUER however has a special featuring for our companions in the pharmaceutical sector.
For starters, we are an set up corporation that has been functioning for pretty much 60 decades. All of these years of encounter suggest that we are perfectly versed in offering custom made system answers to our partners.
Next, we are neither way too massive nor much too compact. Our medium corporation sizing suggests that we can reply flexibly to the wants of our consumers – like we did throughout the coronavirus pandemic with our lipid nanoparticle technological know-how – whilst even now obtaining the manpower to supply methods at speed.
Our third special energy is that we deliver all of our techniques in-household at our headquarters in Berlin. Our on-website generation allows us to swiftly adapt our present techniques to our customers’ needs employing our own CNC output, tools assembly line and program progress crew.
For instance, if a buyer desires a exclusive valve to entire a method that is not offered on the marketplace, our design crew can style and design the necessary portion in accordance to their needs.
Following style, the section can then be developed by our CNC output and connected to the program. Our software workforce can then plan any functions necessary to combine the valve. This overall course of action can speedily be carried out in-residence, depending on the time demands of our shoppers.
To summarize all of this – KNAUER can offer out-of-the-box, custom made remedies for the pharmaceutical sector as and when necessary.

An illustration of a custom made process intended by KNAUER – this technique utilizes 16 pumps for the purification of monoclonal antibodies.
mRNA vaccines have at this time been producing headlines – how do KNAUER help in their creation?
The most suited delivery format for mRNA-dependent vaccines is lipid nanoparticles (LNPs). LNPs encapsulate the mRNA made use of in coronavirus vaccines and the resulting lipid capsule protects the fragile mRNA from degradation, expanding integration into the patient’s cells.
The mRNA encapsulation procedure utilizes Impingement Jets Mixing technological know-how, where by two streams (a single made up of lipids and a different containing an aqueous remedy of mRNA) collide at significant velocity in a jet mixing chamber – it is this move that KNAUER’s technologies is included in.
With our knowledge in systems engineering and high-pressure dosing, we were being equipped to offer you a significant pharmaceutical maker the know-how they expected for the large circulation production of lipid nanoparticles.
Our Impingement Jets Mixing Skids have proven exceptional overall performance in the significant-scale output of mRNA encapsulated in lipid nanoparticles for use in coronavirus vaccines. These are now getting applied globally in identical applications.
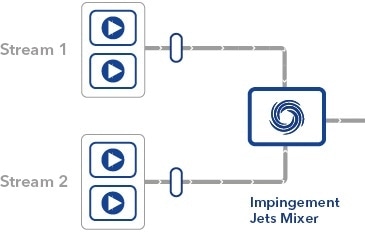
Schematic diagram exhibiting the mixing procedure taking place inside an IJM procedure.
Are there any breakthroughs or developments that KNAUER is pioneering that you are particularly psyched about?
The Impingement Jets Mixing (IJM) Skids we protected in the last query are what excites me most appropriate now.
Right before the coronavirus disaster, products for the production of LNPs only existed at a laboratory scale, indicating the largescale production of mRNA encapsulated in LNPs was not nevertheless doable.
The coronavirus disaster meant that technology that could be applied in the massive scale creation of mRNA-based mostly vaccines out of the blue became urgently important. This posed a obstacle as the massive scale manufacturing of these units experienced not nevertheless been carried out, and the lab-scale generation of LNPs was not quickly scalable.
To clear up this dilemma, we combined various lab-scale models in parallel to produce our IJM Skids, which could then be utilised for LNP production at a large scale. This appears like a simple resolution, and, in theory, it is. Nevertheless, it necessary a significant degree of finetuning and knowledge to put into action this according to the regulations and needs of the biopharmaceutical field.
Our existing gadgets experienced to be adapted for pharmaceutical manufacturing, which included the style of personalized manifolds for inlets and retailers, stream route optimization, layout adaptation to meet up with clean up home C needs and the provision of interfaces for our customers’ own PLC systems.
We are proud to have played a position in earning historical past with coronavirus mRNA vaccines, staying the initial instance of the use of mRNA-dependent LNP vaccines. We’re even much more proud to have carried out all of this under remarkable time pressure, helping to deliver a coronavirus vaccine when the entire world desired it most.
KNAUER’S IJM Skids have performed these types of a essential function in the creation of mRNA coronavirus vaccines in Germany that the German Chancellor herself frequented the enterprise in September 2021.
What forms of assignments in the pharmaceutical market has KNAUER been concerned in?
Thanks to the rigid confidentiality needed by the biopharmaceutical sector, we can’t identify any names. We are, on the other hand, enthusiastic to be performing with a person of the most significant providers of mRNA coronavirus vaccines.
Our 1st job with this spouse was just one of the most thrilling assignments for our engineering-to-purchase company to date. This is because of the time pressure and the actuality that the technique we were being establishing had never ever been generated just before.
The urgent have to have for the generation of mRNA vaccines intended that a common challenge move – with preparing, engineering, provide and get phases – could not be made use of. Manufacturing started quickly subsequent the first request, even though our engineers intended the procedure in parallel to creation with our buyer.
Thanks to the fast-relocating specifications of the task, we had to remain adaptable. In some cases do the job we had finished just one day needed to be reversed or modified the subsequent day. Pursuing a few months of extremely concentrated growth, we ended up happy to see the method in output.
It is crucial to point out that our choices for the pharmaceutical business go further than our IJM Skids. We also provide finish alternatives for the purification of other active pharmaceutical elements (APIs).
We are often approached by customers who know their goal API and know how to make it but deficiency the skill to purify it – this is the place KNAUER can support.
When approached by this kind of a shopper, we assist them from the bottom up. We commence with a column screening, then build a system and improve it at the lab scale. Applying these final results, we can then run the purification making use of our simulated shifting mattress (SMB) process, which lets for constant purification.
Pursuing this scale-up, our shopper can then start out producing API at a pilot scale.
How does KNAUER assistance GMP compliance for pharmaceutical manufacturers?
KNAUER offers expertise and means to pharmaceutical makers to aid them carry out GMP-compliant pharmaceutical manufacturing. Our consumers typically have unique requirements for the KNAUER hardware that they are intrigued in, and these are typically defined in their unique Person Necessity Technical specs (URS).
We examine the URS with our shoppers and support ensure that their regulations continue to be up to day, encouraging manufacturers deliver products and solutions that fulfill their conclusion users’ necessities and adhere to all expected basic safety specifications.
Our GMP products and services are based on our hardware and software solutions. Product basic safety is supported by documentation on solution conformity and the compliance of materials applied as wetted elements.
Quality manage is ensured by set up and operational qualification, as properly as performance verification. In addition, a manufacturing facility acceptance examination (Extra fat) and a aspect acceptance take a look at (SAT) are regular GMP products and services.
We also put a fantastic emphasis on private teaching, which includes software package, hardware, routine maintenance and assistance education offers.
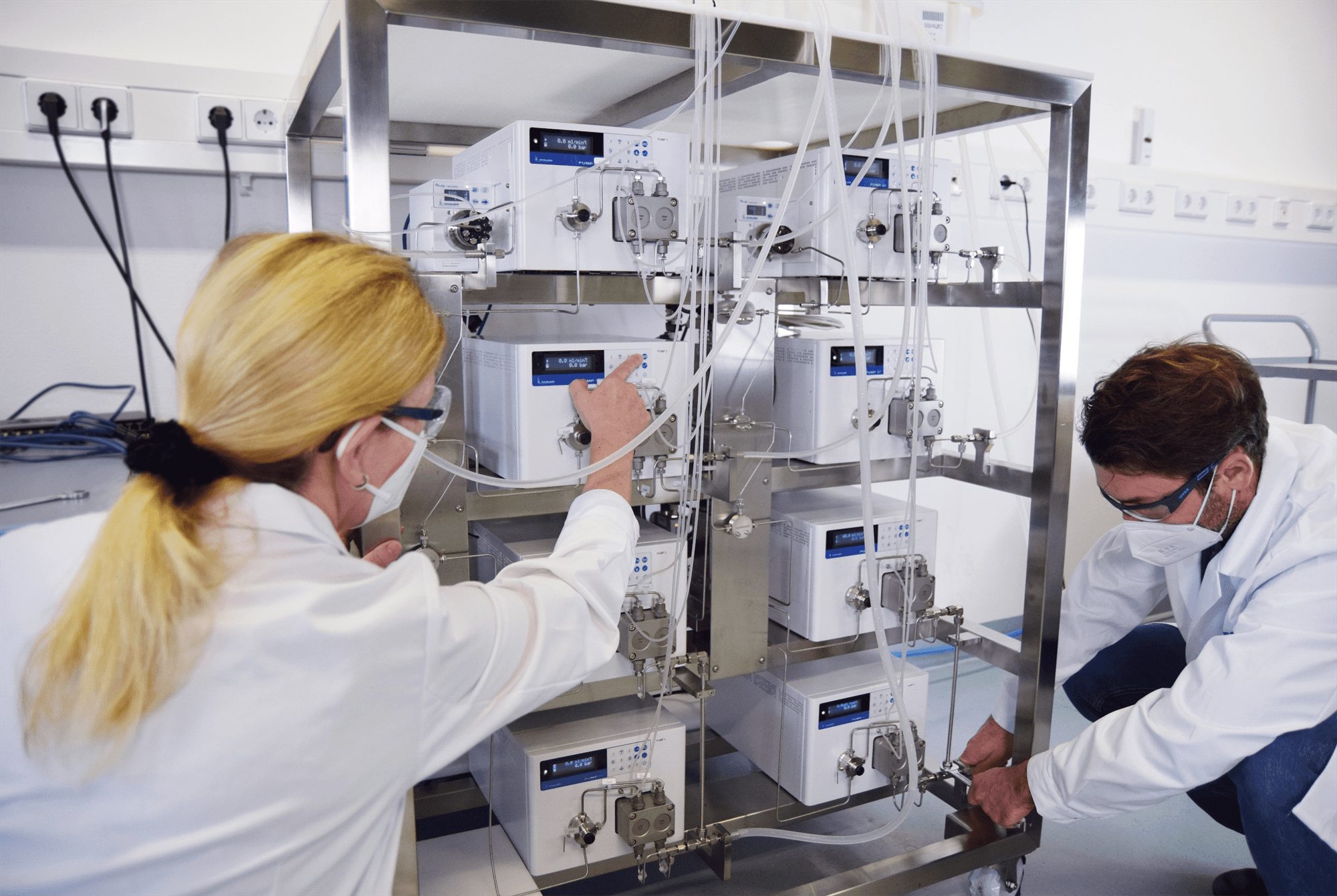
Set up and testing of an IJM Skid for the encapsulation of API in lipid nanoparticles
What are some of the new troubles that confront the pharmaceutical marketplace in the wake of the COVID-19 disaster, and how will KNAUER enable tackle them?
From the COVID-19 crisis, we have realized to be open to new systems and that, for us, currently being quick and adaptable are the keys to results.
In a even bigger perspective, there is a possibility that the coronavirus disaster has accelerated a excellent change in the vaccine landscape – a migration to mRNA-centered vaccines appears to be on the horizon.
mRNA vaccines have the exceptional edge that they can be rapidly modified in reaction to virus mutations, and this reward could see them commencing to dominate the discipline in the coming a long time.
LNP-encapsulated mRNA technologies does not end there both – we expect to see the technology staying employed in gene therapy, although LNPs, in general, can be employed for the shipping of other fragile APIs. We are at the starting of a new period in medication and we’re very pleased to be at the forefront.
We’re thrilled to be portion of this revolution and glance ahead to supporting travel this clinical revolution with our tailor-manufactured methods for pharmaceutical processing and generation.
How does KNAUER intend to work along with customers in a article-COVID earth?
We intend to do the job along with individuals as we did prior to. For us, shut collaboration with our consumers is the key to establishing items and units that our clients are definitely joyful with.
We have a committed team of professionals from hardware design to high quality gurus accessible in the course of the finish layout process, and these gurus are on hand to aid our prospects with the know-how they need to have to uncover the greatest alternative to their challenges.

About Dr. Paul Pietsch
Dr. Paul Pietsch studied Foods Technological know-how at the Anhalt College of Applied Science ahead of likely on to complete a chemistry Ph.D. at the Technical University of Berlin. Following completion of his doctorate, Dr. Pietsch joined the group at KNAUER as a solution manager for their continual chromatography systems. Immediately after 3 and a 50 percent many years in this role, he moved on to grow to be the head of engineering solutions throughout the total company.
About KNAUER Wissenschaftliche Geräte GmbH
KNAUER Wissenschaftliche Geräte GmbH is a center-sized business that has been developing, producing and distributing laboratory devices about the earth because 1962.
With much more than 130 staff members, Knauer is a single of the nicely-set up companies of HPLC devices, SMB programs, and osmometers. Solution portfolio includes incredibly compact HPLC answers, UHPLC units for substantial-resolution examination, preparative HPLC devices, procedure LC products for the purification of substances in the kilogram scale, autosamplers, column thermostats, degassers, detectors, dosing pumps, eluent mixers, flowmeters, LC columns, and equipment and spare parts, among other individuals.
The source of Knauer’s achievements is several world’s firsts that have won much more than 20 awards for innovation.






